
DISCOVER WHAT’S POSSIBLE WITH FILSPARI®
It’s time to see a difference in your treatment
Upgrade to FILSPARI, an innovative IgA nephropathy (IgAN) treatment for adults proven to powerfully lower proteinuria and preserve kidney function by working at the site of damage in the kidneys.

Not actual patients.
Proven long-term IgAN treatment
FILSPARI has been studied in adults with IgAN for over 2 years. The PROTECT Study compared FILSPARI and irbesartan, a type of blood pressure medication called an angiotensin receptor blocker (ARB), often used to treat IgAN.
FILSPARI does not suppress your immune system
FILSPARI is not a steroid. It is an FDA-approved IgAN treatment that does not suppress your immune system.


HOW FILSPARI WORKS
The single pill that works in 2 ways
With IgAN, a number of undesirable processes involving endothelin-1 (ET-1) and angiotensin II (Ang II) take place in the kidneys. ET-1 and Ang II are known to damage the kidneys by:
- Weakening the kidneys' filters
- Allowing increased amounts of protein to spill out of the blood into the urine (proteinuria)
FILSPARI takes a different approach to treating IgAN by targeting both ET-1 and Ang II in the kidneys. Blood pressure medicines that are used to treat IgAN only target Ang II.

SEE THE RESULTS
Powerfully lower proteinuria
The PROTECT Study, a clinical trial of 404 people with IgAN, compared FILSPARI with irbesartan. Here's what was learned from that study:
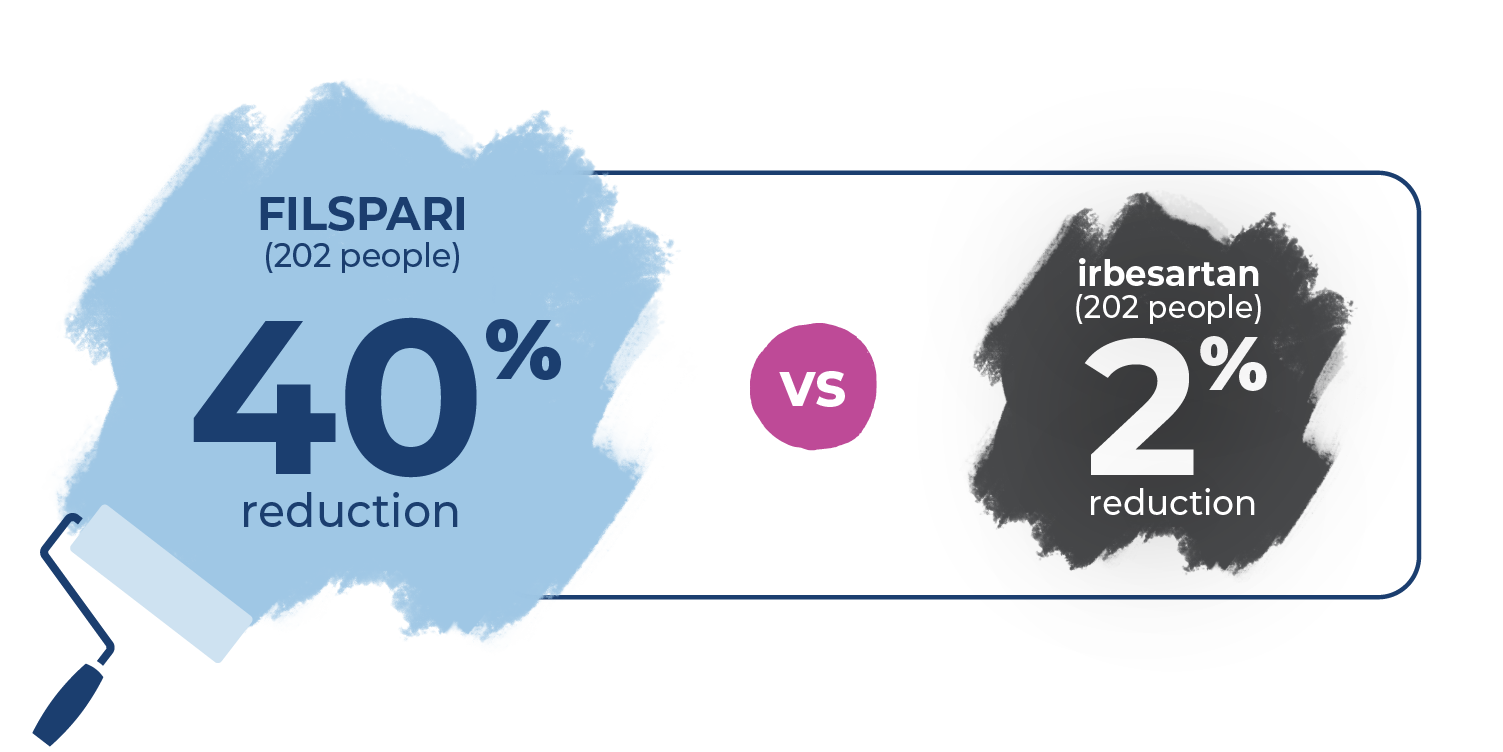
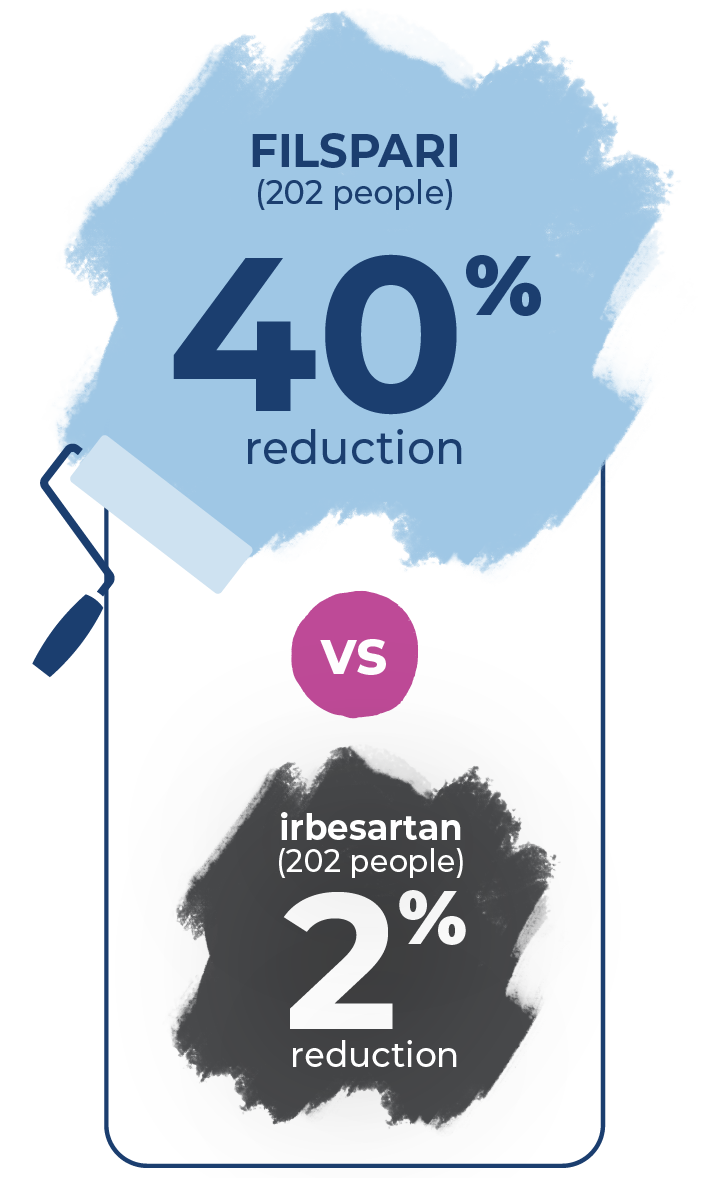
People taking FILSPARI and irbesartan started the study with proteinuria levels at 1.2 grams per gram (g/g). At 36 weeks, FILSPARI reduced proteinuria by 45% to 0.7 g/g. Irbesartan reduced proteinuria by 15% to 1.0 g/g.
FILSPARI sustained significantly lower proteinuria through 2 years: 40% less proteinuria (0.7 g/g) compared to only 2% (1.2 g/g) with irbesartan.



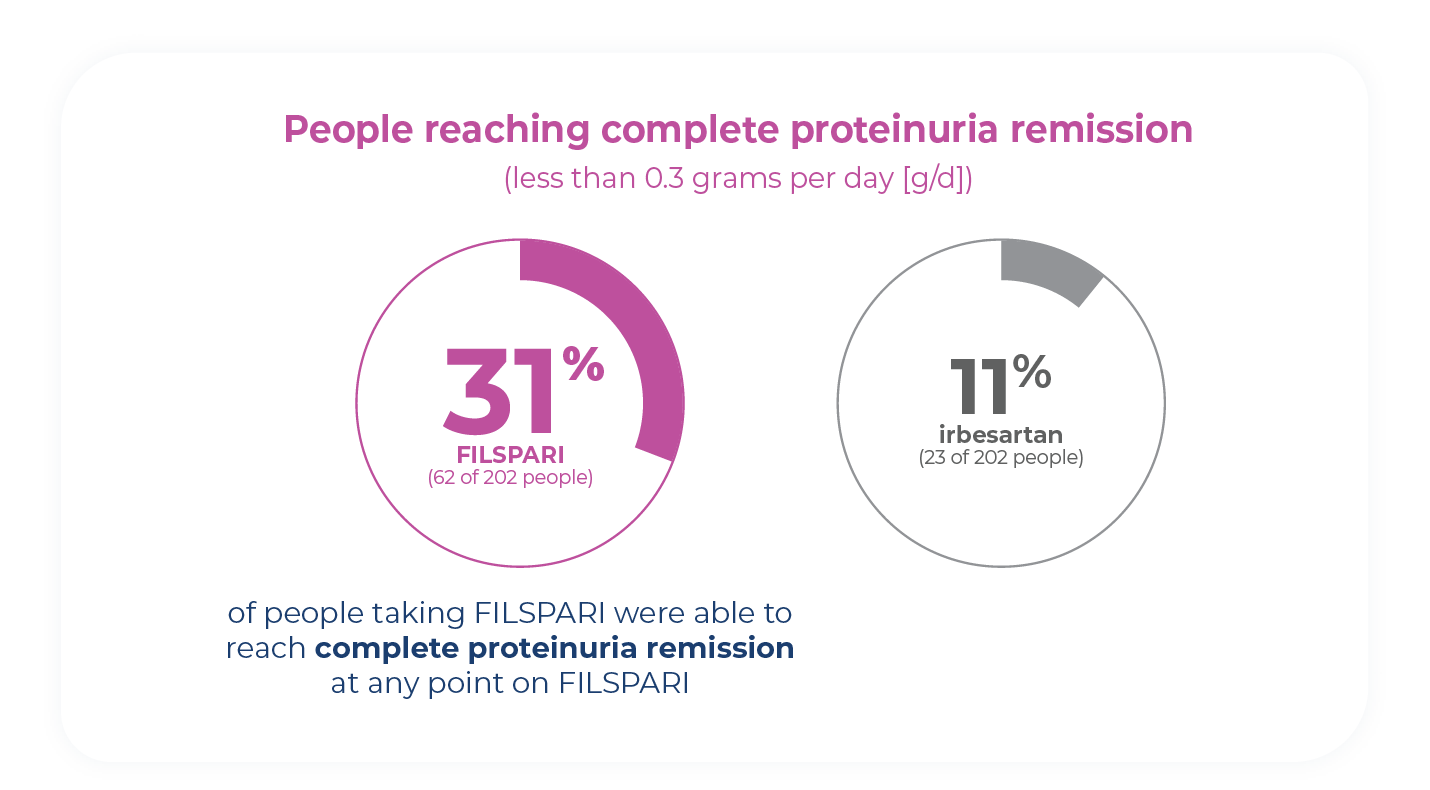
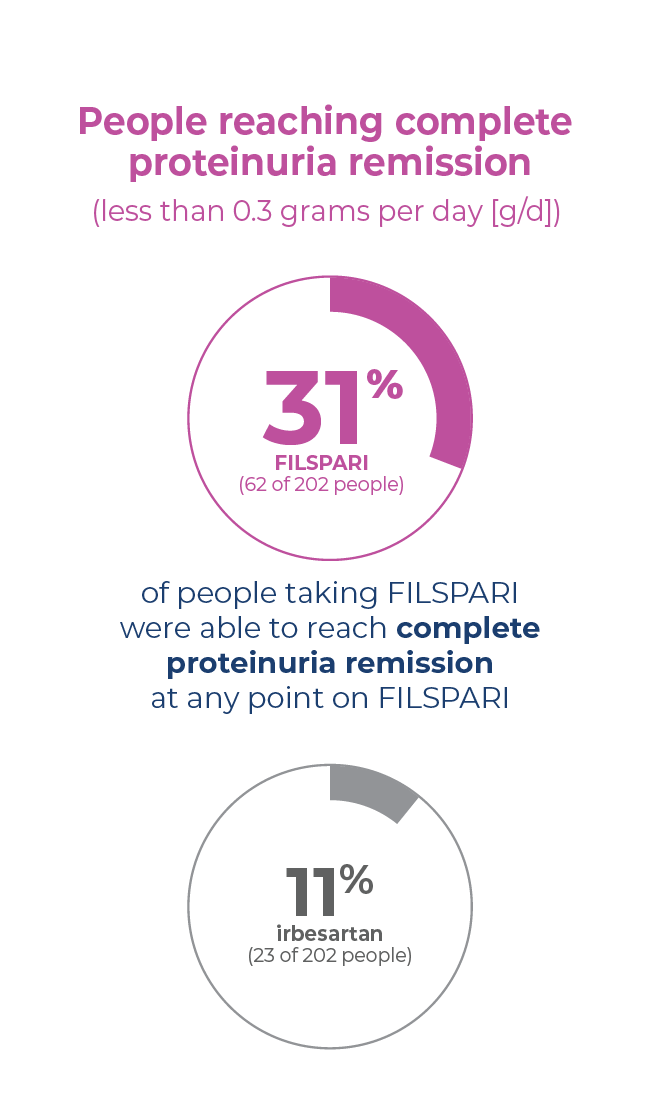
Proteinuria remission is possible
Complete proteinuria remission in IgAN occurs when you have little to no protein detected in your urine. During the clinical study, more people taking FILSPARI reached complete proteinuria remission compared to people taking irbesartan.
Preserve kidney function for longer
Your estimated glomerular filtration rate (eGFR) measures how well your kidneys are functioning. The less your kidney function declines each year, the better.
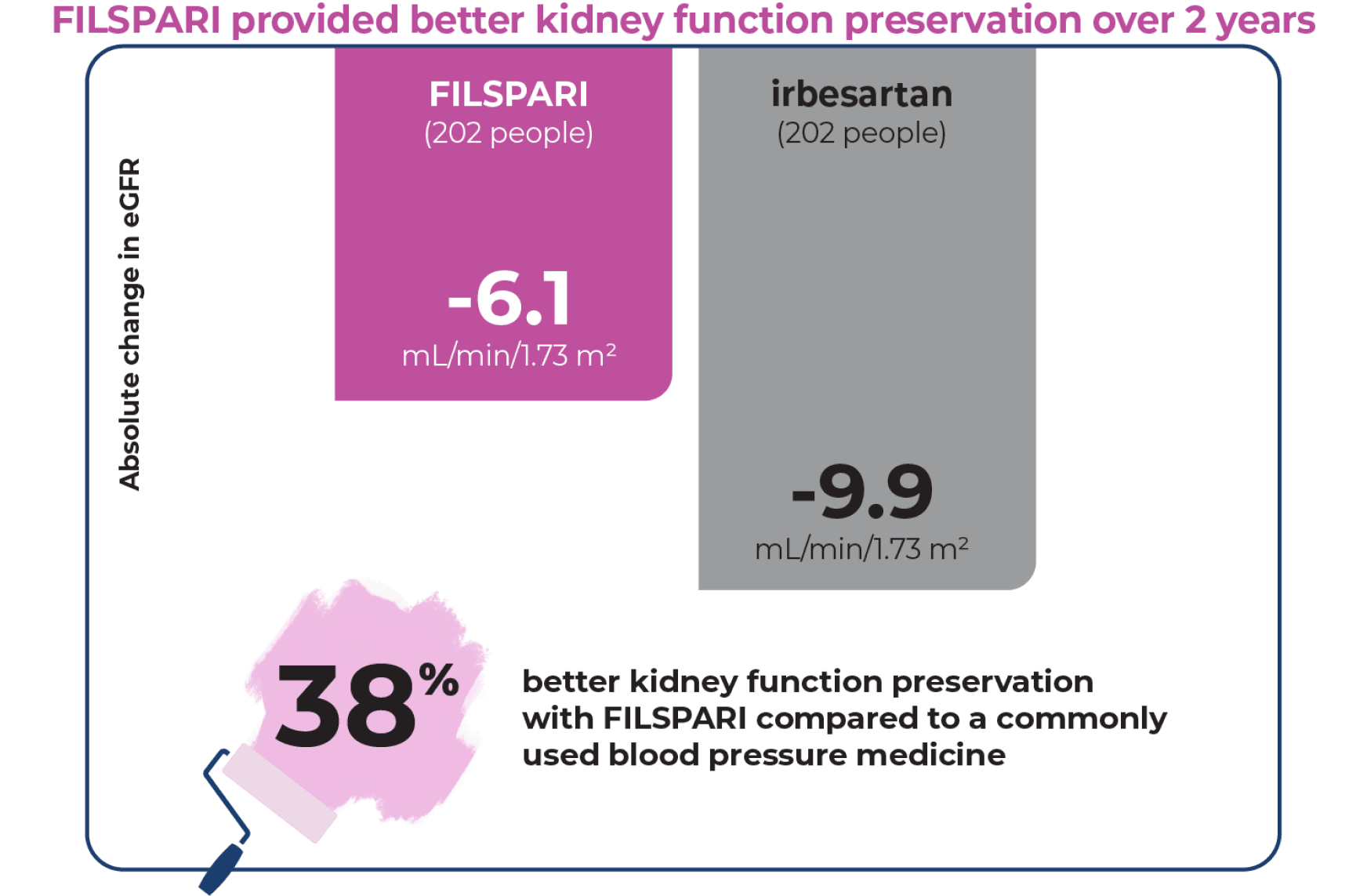
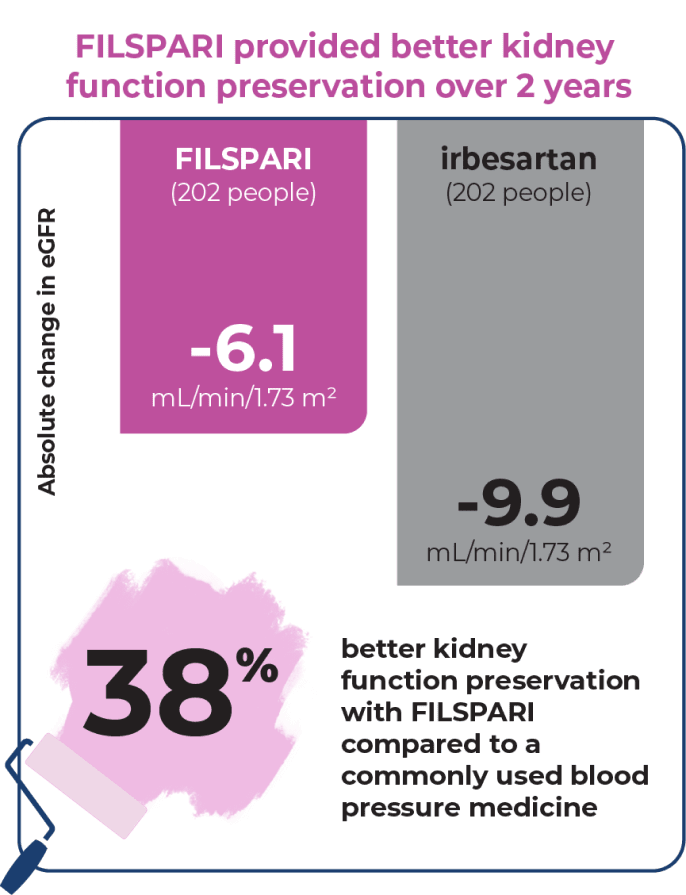
At the beginning of the 2-year study, eGFR was 57 mL/min/1.73 m2 for people taking FILSPARI and irbesartan. As the study progressed, the benefit of FILSPARI over irbesartan improved from Year 1 (+1.9) to Year 2 (+3.8).
FILSPARI significantly slowed the loss in kidney function
Through the 2 years of the clinical study, the average decrease in kidney function was only -3.0 mL/min/1.73 m2 per year with FILSPARI compared to -4.2 mL/min/1.73 m2 per year with irbesartan.
Most did not need to add a steroid

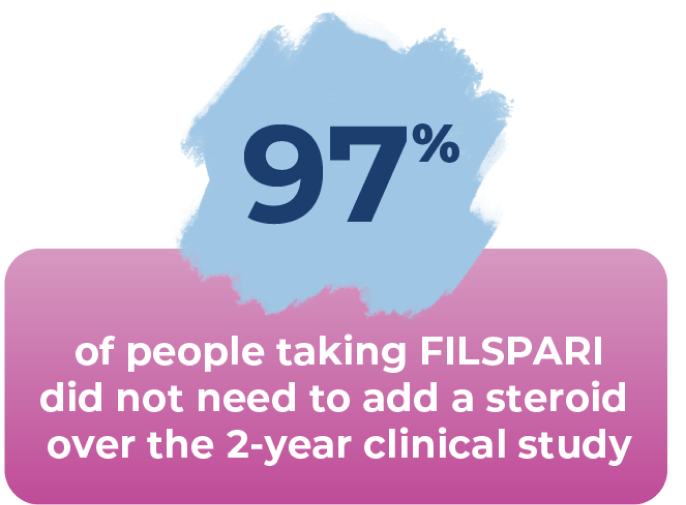
During the study, steroids were added on to treatment only if needed. People needed steroids less frequently with FILSPARI than with irbesartan.
Of the 202 people taking irbesartan, 9% added a steroid, while only 3% of the 202 people taking FILSPARI did.




The PROTECT Study evaluated the effects of FILSPARI in adults with IgAN
The main focus of the PROTECT Study was to measure the change in proteinuria and eGFR from the beginning of the study through 110 weeks of treatment. Half of the people in the study were given FILSPARI and the other half were given irbesartan.
Participants in the trial had a wide range of proteinuria levels prior to treatment, with levels of at least 1 g/d, even after being treated with an angiotensin-converting enzyme (ACE) inhibitor or ARB (blood pressure medications).
At the beginning of the study, participants' proteinuria levels averaged 1.2g/g.
The average eGFR of those studied was 57 mL/min/1.73 m2, indicating a mild-to-moderate loss of kidney function. To participate in the study, patients needed to have an eGFR of at least 30 mL/min/1.73 m2.
Participants were aged 18 to 76 years with an average age of 46 years. 70% were male.
POTENTIAL SIDE EFFECTS
What are the potential side effects of FILSPARI?
Tell your doctor if you have any side effect that bothers you or that does not go away.
Possible serious side effects:
Your doctor will order certain tests on a routine basis to monitor your health, help you manage any side effects, and adjust your treatment plan as necessary. Certain possible side effects that your doctor will be monitoring may include:
Monitoring for liver problems: FILSPARI can cause changes in liver tests. Liver failure was not observed in people treated with FILSPARI in the clinical study, but some medicines that are like FILSPARI can cause liver failure. Learn more about the FILSPARI REMS Program.
Blood tests will be done:
- Before you start treatment with FILSPARI
- Monthly for the first 12 months
- Every 3 months after that while taking FILSPARI
Your doctor may temporarily stop or permanently stop treatment with FILSPARI if you have changes in your liver tests.
Stop taking FILSPARI and get medical help right away if you develop any of the following signs: nausea or vomiting, pain on the upper right side of your stomach area, tiredness, loss of appetite, yellowing of your skin or the white part of your eyes (jaundice), dark “tea-colored” urine, fever, or itching.
Birth defects: Do not take FILSPARI if you are pregnant, plan to become pregnant, or become pregnant during treatment.
The FILSPARI REMS Program includes measures to prevent pregnancy while taking FILSPARI. Learn more about the FILSPARI REMS Program.
Patients who can become pregnant must use effective birth control:
- Before starting treatment with FILSPARI
- During treatment with FILSPARI
- For 1 month after stopping FILSPARI, because the medicine may still be in your body
Patients who can become pregnant must have a negative pregnancy test:
- Before starting FILSPARI
- Every month during treatment
- 1 month after stopping FILSPARI
Review the list of options for acceptable birth control in the Medication Guide and discuss with your doctor or gynecologist which options work best for you.
Other possible serious side effects:
Low blood pressure: Low blood pressure is common during treatment with FILSPARI and can also be serious. Tell your doctor if you feel dizzy, light-headed, or faint. Stay hydrated while taking FILSPARI.
Increased potassium in your blood: This is common during treatment with FILSPARI and can also be serious. Your doctor will check your potassium blood level during treatment with FILSPARI.
Worsening of kidney function: Your doctor will check your kidney function during treatment with FILSPARI.
Fluid retention: FILSPARI can cause your body to hold too much water. Tell your doctor right away if you have any unusual weight gain or swelling of your ankles or legs.
The most common side effects seen in the FILSPARI clinical study are shown in the table below.*
These are not all the possible side effects of FILSPARI.
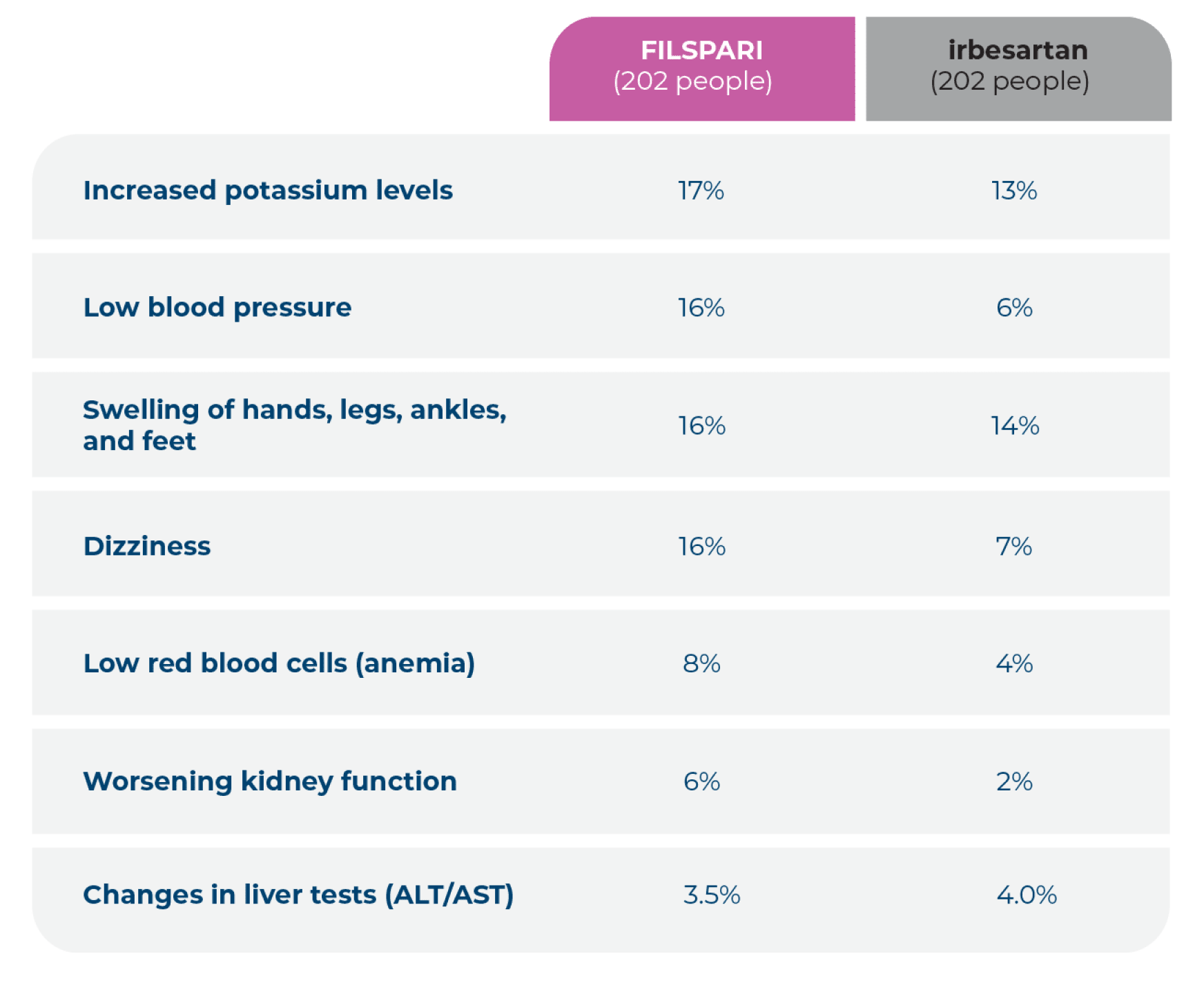

*These side effects were experienced by at least 2% of those taking FILSPARI. Side effects were recorded over a median time of 110 weeks on treatment.
ALT=alanine aminotransferase; AST=aspartate aminotransferase.
Starting treatment
Learn more about taking FILSPARI, what to expect with treatment, and important considerations.
FILSPARI Brochure
A downloadable brochure with useful information about FILSPARI, a treatment to lower proteinuria and preserve kidney function.

